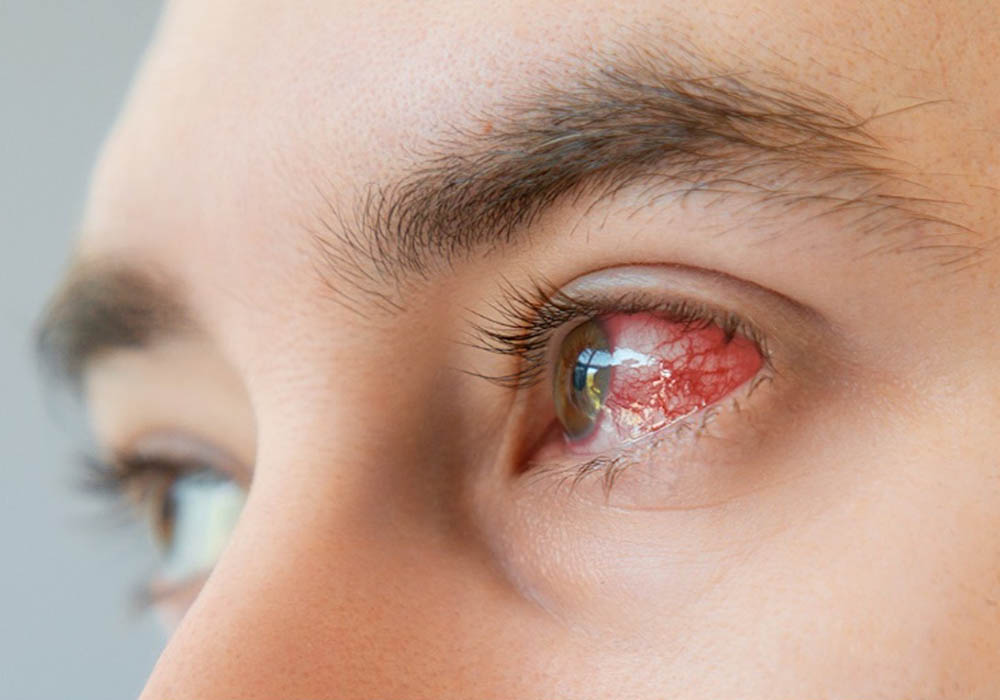
More than 40% of patients receiving antibody-drug conjugates (ADCs) will experience ocular toxicities during treatment, ONS members Caroline Clark, MSN, APRN, OCN®, AGCNS, EBP-C, and Ikuko Komo, MSN, CNS, NP, AOCNS®, AOCNP®, reported in an article in the April 2024 issue of the Clinical Journal of Oncology Nursing.
In their article, Clark (who is also ONS’s director of evidence-based practice and inquiry) and Komo presented the current evidence for what nurses need to know about the incidence, presentation, and prevention and management strategies for ADC-related ocular toxicities in patients with cancer.
ADCs’ Ocular Effects
Clear reasons for the drugs’ ocular toxicities are not yet established, but theories include “expression of the target antigen in healthy ocular tissue, the type of cytotoxic payload used in the conjugate, the type of linker, the vascularity of the eye, and the rapid cell division in the eye,” Clark and Komo said. Depending on the agent, some of the most common ocular toxicities are:
- Conjunctival and corneal reactions
- Dry eye
- Keratopathy
- Visual impairments
Prevention
At the time of publication, Clark and Komo said that enfortumab vedotin-ejfv, mirvetuximab soravtansine-gynx, and tisotumab vedotin-tftv had the highest risk of ocular toxicities. All patients receiving those drugs should adhere to baseline and routine eye exams from an ophthalmologist or optometrist, and patients who are taking tisotumab vedotin-tftv or mirvetuximab soravtansine-gynx require steroid eye-drop prophylaxis.
Presentation and Assessment
See the sidebar for the signs and symptoms of the most common ocular toxicities and nursing management strategies. Following proper eye hygiene is essential for both prevention and management, including:
- Washing hands before using eye drops
- Avoiding touching or rubbing the eyes
- Avoiding sharing towels, linens, and other materials that may come in contact with eyes to prevent cross-contamination
If ocular effects such as dry eye and vision changes occur, nurses should monitor the impact on patients’ activities like reading, driving, and using a computer.
Patient Education
Prior to treatment, nurses should advise patients about the potential for ocular toxicities and prepare them for the possibility of dose reductions or discontinuations. Throughout treatment, nurses should provide patient education about required eye examinations, eye hygiene and symptom prevention, use of eye drops, and avoidance of eye irritants, including contact lenses, Clark and Komo said.
For specific ADCs, instruct patients receiving mirvetuximab soravtansine-gynx on how to use lubricating eye drops four times daily and patients receiving tisotumab vedotin-tftv on using eye drops at least once daily and for 30 days after their final dose.
Tisotumab Vedotin-Tftv Administration Considerations
Tisotumab vedotin-tftv’s prescribing information advises that eye cooling during administration and the use of vasoconstrictor eye drops immediately prior to drug infusion can help to minimize toxicity by reducing ocular blood flow, Clark and Komo said.
Patients should bring a topical corticosteroid and vasoconstrictor drops to their treatment visit and use them directly before the infusion. Patient- or institution-supplied eye masks or cold packs should be applied 10 minutes prior to infusion, 30 minutes during infusion, and 20 minutes postinfusion, for 60 minutes total. “Rotating eye masks or cold packs as needed throughout the infusion ensures the eyes remain cold,” Clark and Komo said.
For more information about ADC-related ocular toxicities, including a sample standardized institutional workflow, refer to the full Clinical Journal of Oncology Nursing article by Clark and Komo.
For a more in-depth look at cancer-related ocular toxicities, listen to the Oncology Nursing Podcast Episode 303: Cancer Symptom Management Basics: Ocular Toxicities on your favorite podcast platform or by using the player below.