Testing for molecular biomarkers has become a mainstay in determining treatment approaches for advanced-stage non-small cell lung cancer (NSCLC). Typically, next-generation sequencing identifies any potential genomic variants that are associated with oncogenic driver events and can be targeted with oral tyrosine kinase inhibitors (TKIs).
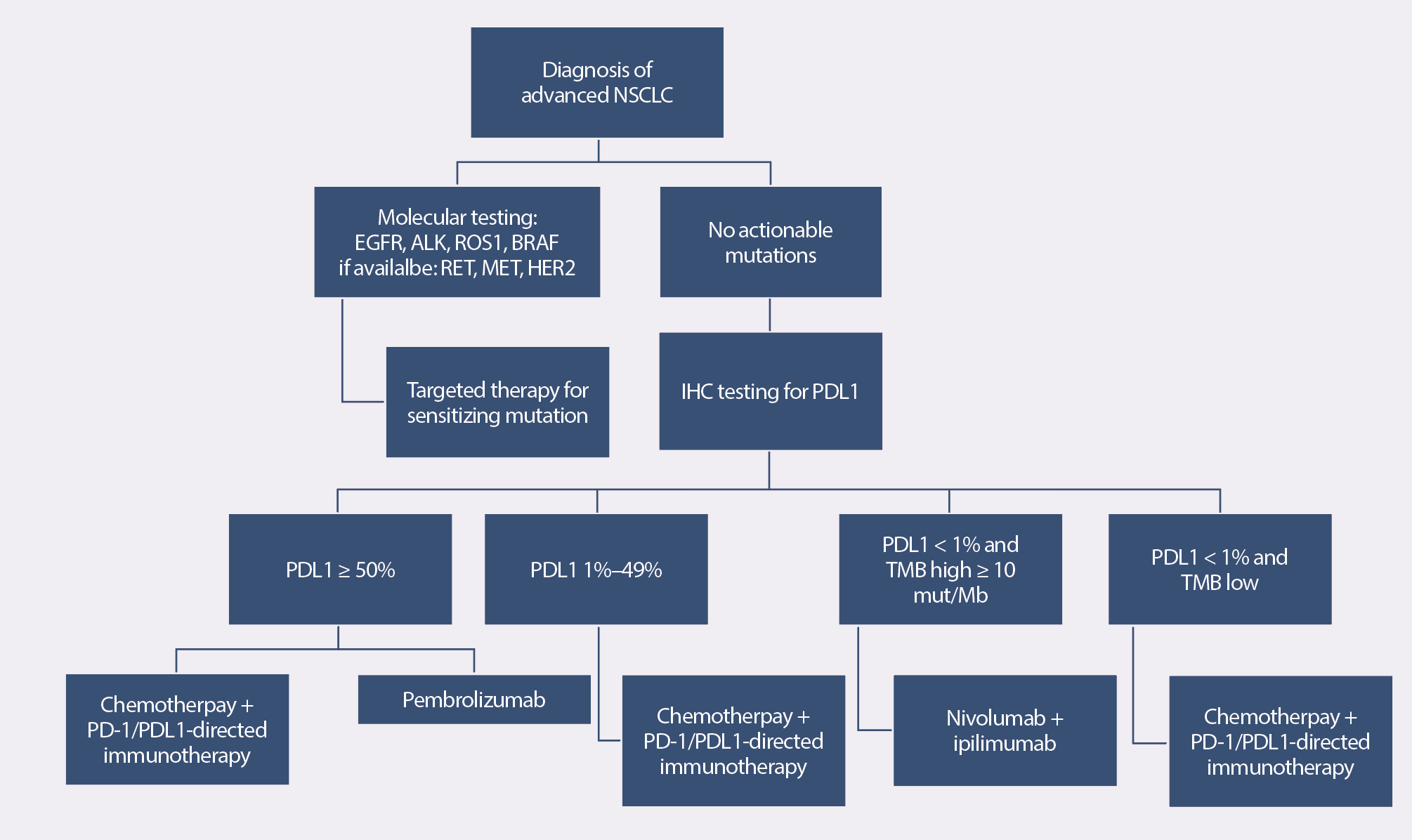
Treatment Algorithm for Stage IV Non-Small Cell Lung Cancer
Biomarkers Predict Response to TKIs
The first targeted agent approved for NSCLC was gefitinib, an oral epidermal growth factor receptor (EGFR) TKI. In the drug’s initial clinical trial, results suggested that a specific demographic of primarily Asian, female, and never smokers with adenocarcinoma experienced the greatest response. As researchers conducted additional studies to investigate the differential responses, they discovered two sensitizing EGFR variants: exon 19 and exon 21 point mutation L858R. Subsequent studies confirmed the presence of EGFR variants in approximately 15% of patients with NSCLC who are of European descent and 35% of Asian descent. Together, those account for 80%–90% of responses to EGFR TKIs.
Researchers have continued to identify additional oncogenic driver variants for NSCLC: anaplastic lymphoma kinase gene (ALK), c-ros oncogene 1 (ROS1), v-raf murine sarcoma viral oncogene homolog B1 (BRAF), rearranged during transfection (RET), human epidermal growth factor receptor 2 (HER2), and mesenchymal-to-epithelial transition (MET). In a landmark report, the Lung Cancer Mutation Consortium demonstrated though multicenter genomic testing that up to 50% of NSCLC and 64% of adenocarcinomas have a driver mutation.
Implications for Testing and Treatment
Because of that widespread prevalence, national organizations such as the National Comprehensive Cancer Network, American Society of Clinical Oncology, College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology have issued guidelines that call for routine biomarker testing for EGFR, ALK, and ROS1 variants.
A treatment algorithm for patients in whom testing reveals an actionable variant is included in the sidebar. If testing doesn’t identify a driver variant, patients are tested for PDL1 expression, and treatment is determined by the overall level of expression and tumor mutational burden.
The Future of NSCLC Treatment
The advent of targeted therapies in NSCLC has offered many patients an option that produces a durable treatment response with a better-tolerated side-effect profile when compared to conventional chemotherapy. However, despite those breakthroughs, tumor cells are smart, and many eventually become resistant to the targeted agent. Identifying resistance mechanisms in advanced NSCLC is the next challenge for science and medicine to overcome.






