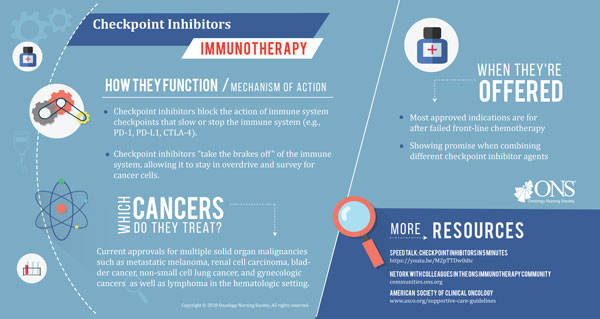
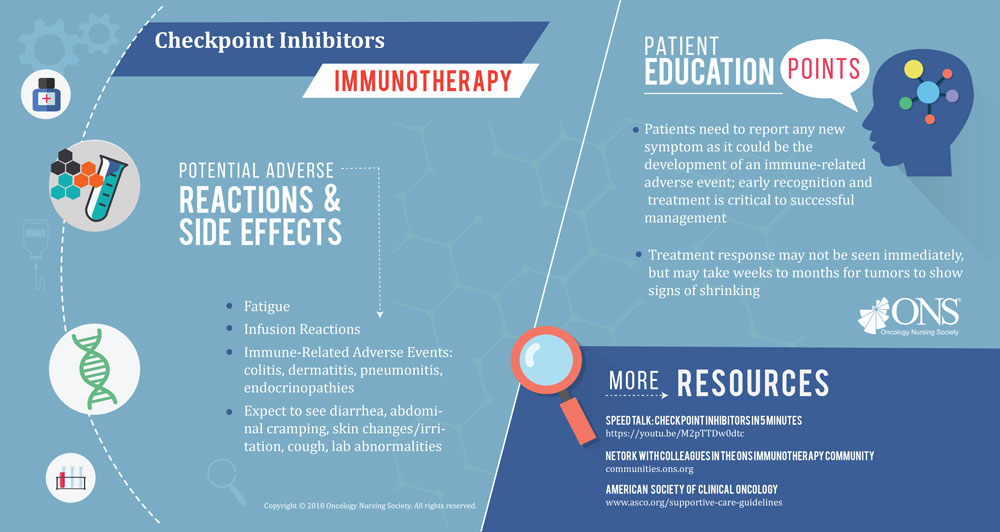
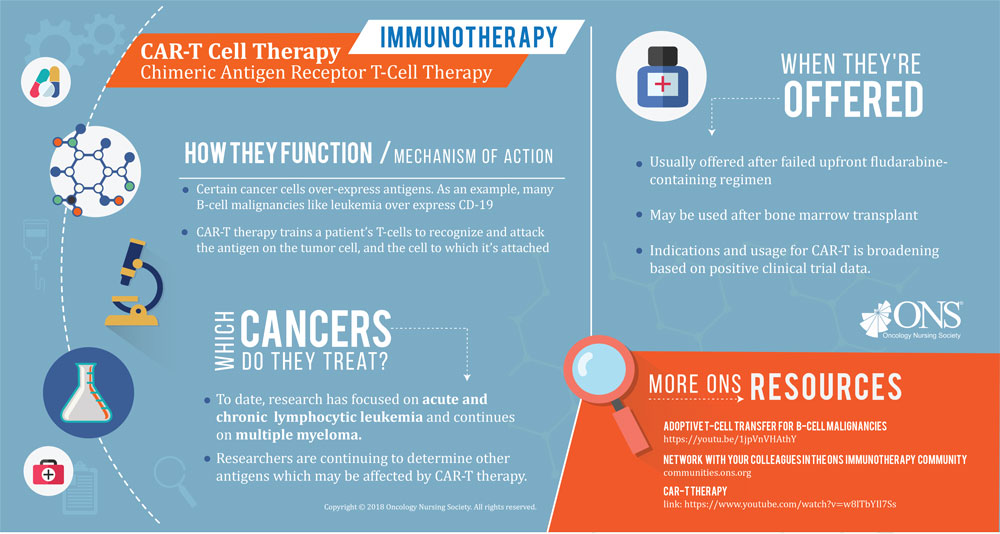
Immunotherapy is one of the fastest-evolving areas of oncology to date. Previously, it could take years for some cancers to see new treatment options; today, the U.S. Food and Drug Administration (FDA) is approving new immuno-oncology agents or new indications for those agents every few weeks.
It’s a boon and a challenge to medical professionals. On the one hand, potentially life-changing treatments are making way to patients who need them—patients who have exhausted first-line treatments and now have limited options. On the other hand, healthcare professionals may struggle to stay current with the emerging trends, cutting-edge science, and evolving treatment plans for their patients.
A need is emerging in all facets of immunotherapy and oncology. Whether your work revolves around benchside science, bedside practice, chairside administration, clinical trial participation, or anything in between, oncology nurses have the opportunity to be frontline caregivers, educators, and advocates for their patients when it comes to immunotherapy.
Rapid Change Brings New Treatments
In 2016 alone, the FDA approved more than 15 immunotherapy treatments with new or existing medications on the market. With names like nivolumab, atezolizumab, pembrolizumab, and avelumab, immunotherapy drug names can often carry the “-mab” or “-ib” suffix (although some "-ib" drugs are targeted agents). Although this isn’t true for every immunotherapy treatment—most notably treatments like T-Vec and CAR-T—this simplification can help. When products are being approved for new indications or interventions at the rate of more than one per month, it’s important to recognize some of them at a glance.
ONS member Laura Wood, RN, MSN, OCN®, research nurse at the Cleveland Clinic Cancer Center, is no stranger to the rapid changes in immunotherapy. “It’s expanding quickly with broad applications in the United States,” Wood says. “Never before have I seen a single drug, nivolumab, approved in both solid tumor malignancies and hematologic cancers—let alone have a total of nine different FDA approvals in just two years.”

Immunotherapy’s progress has been expedited through FDA policies. New treatment options for traditionally tricky cancer diagnoses are emerging quickly. “Immunotherapy has provided a significant change in the treatment options for advanced urothelial cancer, which has been a very difficult cancer to treat,” Wood cites as one example. “In March 2017, the first approval for avelumab was granted for metastatic Merkel cell carcinoma. We now have another PD-1 inhibitor available in a new cancer with already limited treatment options.”
Kelly Brassil, PhD, RN, AOCNS®, ACNS-BC, ONS member and director of nursing programs at the University of Texas MD Anderson Cancer Center in Houston, says, “One of my colleagues recently mentioned that 10–20 years ago, it would take years between these agents being approved. You had time to digest them and explore them. Now we’re talking about a new agent every few weeks. As soon as things are published, they’re already out of date. Nurses are going to have to be savvy about where they get their information and understand that new concepts are key.”
New Immunotherapy Treatments Bring New Challenges
With the rapid release of new drugs and new indications for drugs that already exist, oncology nurses may find it almost impossible to keep up with new information. However, both Wood and Brassil agree that immunotherapy proficiency and understanding starts with learning new concepts about this unique form of treatment.
“One of the main components of immunotherapy that oncology nurses should be aware of is the unique type and presentation of immune-related adverse events (irAEs),” Wood says. “irAEs may occur early or late. Some side effects may occur within days of drug administration, but don’t resolve quickly like those with chemotherapy. For example, neutropenia and thrombocytopenia have a defined course with a nadir and recovery that’s predictable for most patients who receive a specific chemotherapy drug. This is not the case with immunotherapy.”
“Early-onset irAEs may become severe over several weeks or with subsequent treatment. Late-onset irAEs may occur after months of therapy. Because these side effects don’t occur within the first several months of treatment, they’re often attributed to other etiologies,” Wood says. “Oncology nurses, and all healthcare practitioners, must be aware that any ‘-itis’ should be attributed to immunotherapy until proven otherwise. This concept should drive assessment and intervention strategies.”
Wood notes the importance of understanding novel side effects for immunotherapy that aren’t present with other treatment types. “Oncology nurses should be educated regarding side effects not previously seen with chemotherapy agents. These include hyperthyroidism, hypophysitis, muscle weakness, myositis, and Gillian Barre syndrome. Autoimmune colitis, pancreatitis, hyperglycemia, and diabetes mellitus can also occur with immunotherapies.”
Seeking out new and vetted information about immunotherapy is vital to understanding the needs of patient assessment and which side effects are common to certain immunotherapy drugs. Wood recommends a number of educational resources, including the Clinical Journal of Oncology Nursing and the Society for Immunotherapy of Cancer.
New Challenges Breed New Opportunities
Numerous opportunities are emerging for oncology nurses to step into research and study for clinical practice. With so many unknowns, scientists are recognizing areas of opportunity that could be as complex as studying side effects and late effects to simply following up with patients through the end of treatment.

“I think survivorship and a focus on late effects is going to be critical in the future,” Brassil says. “The truth is that right now, we don’t know what we don’t know. We’ll need to be following these patients in the long run to see what’s happening with them.
“Another thing we’ve noticed that’s becoming a critical need is developing evidence-based patient education about who is eligible for immunotherapy treatments,” Brassil continues. “Immunotherapy is a new, cutting-edge, hot-topic science. Some patients are coming in after reading online, on WebMD, or seeing it on the news, and are asking, ‘Why can’t I have it?’ We need to use evidence to inform those patients about eligibility for immunotherapy that’s based on science.”
The flood of new information may lead to patients and providers to make incorrect assumptions. Brassil notes that the research community will have new questions to ask to support education. “Going forward, we need to ask, ‘How are we using evidence to inform the education of patients around the eligibility for immunotherapy based on science?’” Brassil says.
A recent report from STAT suggested that the number of patients who respond to immunotherapy may be lower than expected. It’s still far too early to tell with any certainty, Brassil says, but she recognizes that there’s real opportunity for research to uncover which patients will benefit from immunotherapy cancer treatments, and why it works for some patients and not for others.
“I think there’s opportunity to be investigating how we support patients through the process of immunotherapy treatments,” Brassil says. “There’s also a need to study how patients across the lifespan respond to immunotherapies, particularly niche populations like pediatric, adolescent and young adult, and older adult patients. We need to see if they’re eligible for new immunotherapy treatments and support research around their outcomes, impacts on fertility, and so on. I think the National Cancer Moonshot could do a great job of honing us in, as a research community, to address many of these needs.”
I think there’s opportunity to be investigating how we support patients through the process of immunotherapy treatments.
The Future of Immunotherapy Treatments
Dispelling rumors and myths about immunotherapy treatments will be vital to ensuring patients have a full grasp and understanding of the potential and outcomes of this treatment option. It’s no small task, but it’s one that will undoubtedly fall to oncology nurses now and in the future.
Oncology nurses across the spectrum will play a key role in education for both patients and colleagues. “Infusion nurses must be educated about irAEs, so they can be asking patients specifically about side effects,” Wood says. “Infusion nurses also need to be aware of irAEs, as they see patients much more frequently than the advanced practice provider or oncologist. For inpatients nurses, APRNs and outpatient nurses can be an excellent resource for information regarding immunotherapy.”
With new information and education evolving daily, Brassil recognizes the need for nurses to step into roles of discovery. “I don’t want nurses to think they’re limited,” Brassil says. “We can really come to the table to develop evidence-based ways to assess side effects, provide supportive care throughout treatment, and uncover the best possible ways to provide patients with the education they need.”
With all the unknowns and developing information surrounding immunotherapy, one thing is absolutely certain: Oncology nurses will play an enormous role in the evolution of these treatments, the identification and management of side effects and late effects, the development of necessary patient education, and so much more. Immunotherapy offers another opportunity for oncology nurses to bring their expertise and perspective to lead the charge for quality, patient-centered care.