
On August 31, 2017, based on data from two recently halted clinical trials, the U.S. Food and Drug Administration (FDA) is issuing a statement to inform the public, healthcare professionals, and oncology clinical investigators about the risks associated with the use of Keytruda (pembrolizumab) in combination with dexamethasone and an immunomodulatory agent (lenalidomide or pomalidomide) for the treatment of patients with multiple myeloma. Keytruda (pembrolizumab) is not approved for treatment of multiple myeloma.
The FDA statement is based on the review of data from two clinical trials (KEYNOTE-183 and KEYNOTE-185) evaluating the use of pembrolizumab combined with other treatments in patients with multiple myeloma. On July 3, 2017, the FDA required that all patients in these trials be discontinued from further investigation with this drug, because interim results from both trials demonstrated an increased risk of death for patients receiving Keytruda (pembrolizumab) when it was combined with an immunomodulatory agent as compared to the control group (see statistical analysis section below). Merck & Co., Inc. was made aware of the issue through an external data monitoring committee recommendation and suspended the trials to enrollment on June 12, 2017.
This statement does not apply to patients taking Keytruda (pembrolizumab) for an approved indication. The safety and efficacy of using Keytruda (pembrolizumab) for approved, on-label uses have been proven. Patients on Keytruda (pembrolizumab) for an approved use should continue to take their medication as directed by their health care professional.
Keytruda (pembrolizumab) is currently approved by the FDA for treatment of:
- Melanoma
- Lung Cancer
- Head and Neck Cancer
- Classical Hodgkin Lymphoma
- Urothelial Carcinoma
- Microsatellite Instability-High (MSI-H) Cancer
Other multiple myeloma clinical trials of Keytruda (pembrolizumab), other PD-1/PD-L1 cancer drugs and other combinations are currently undergoing clinical evaluation. The FDA will be working directly with sponsors of Keytruda and other PD-1/PD-L1 cancer drugs, as well as clinical investigators conducting clinical trials in patients with multiple myeloma, to determine the extent of the safety issue. The agency will communicate any new information to the public as soon as it is able.
Health care professionals and consumers are encouraged to report any adverse events or side effects related to the use of these products and other similar products to FDA’s MedWatch Adverse Event Reporting program by:
- Completing and submitting the report online at MedWatch online voluntary reporting form; or
- Downloading and completing the form, then submitting it via fax at 1-800-FDA-0178.
Statistical Analysis and Findings
Following is a summary of findings from Merck’s clinical trials. A full safety and efficacy analysis based on a June 2, 2017 data cutoff date was conducted for studies KEYNOTE-183 and KEYNOTE-185. A summary of these results is presented below.
KEYNOTE-183
KEYNOTE-183 is a phase 3, randomized, controlled trial of pomalidomide and low-dose dexamethasone with or without pembrolizumab in patients with relapsed and refractory multiple myeloma who had received at least two prior lines of therapy.
Using a data cutoff date of June 2, 2017, a complete evaluation of safety and efficacy was performed. There were 249 randomized patients included in the analysis. The median follow-up was 8.1 months. For overall survival, there were 29 deaths on the pembrolizumab-containing investigational arm and 21 deaths on the control arm. The hazard ratio of the pembrolizumab-containing investigational arm compared to the control arm was 1.61 (95% CI: 0.91, 2.85), increasing the relative risk of death by more than 50% compared to the control arm.
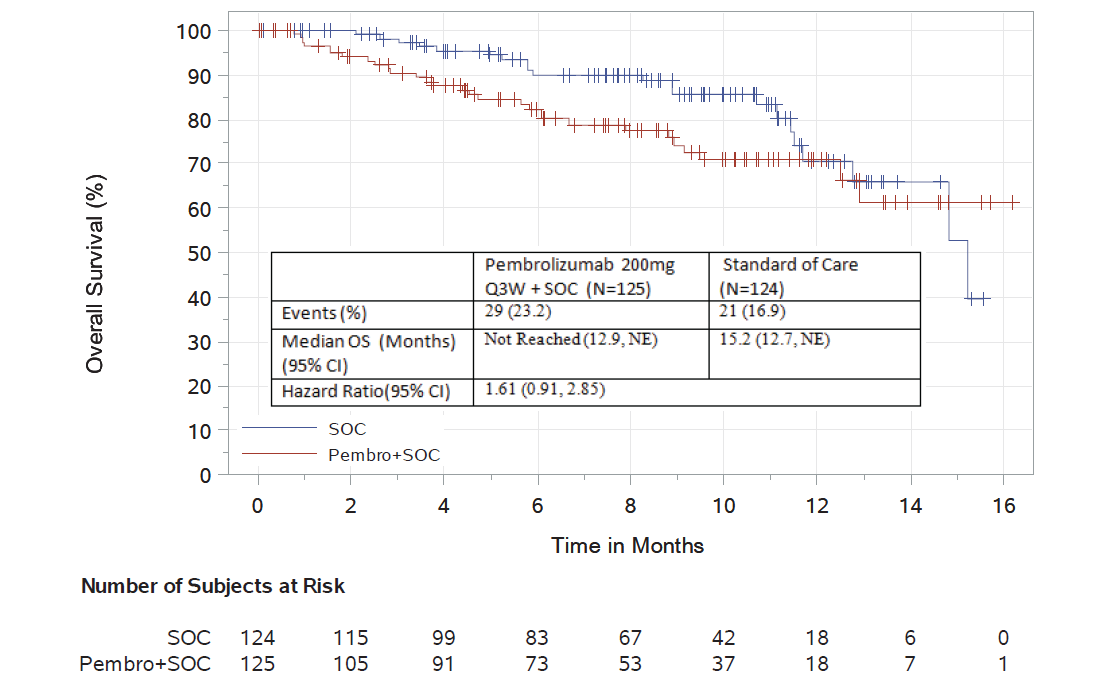
The objective response rate was 34% in the investigational arm compared to 40% in the control arm. In an exploratory time-to-progression analysis, a hazard ratio of 1.14 (95% CI: 0.75, 1.74) was observed.
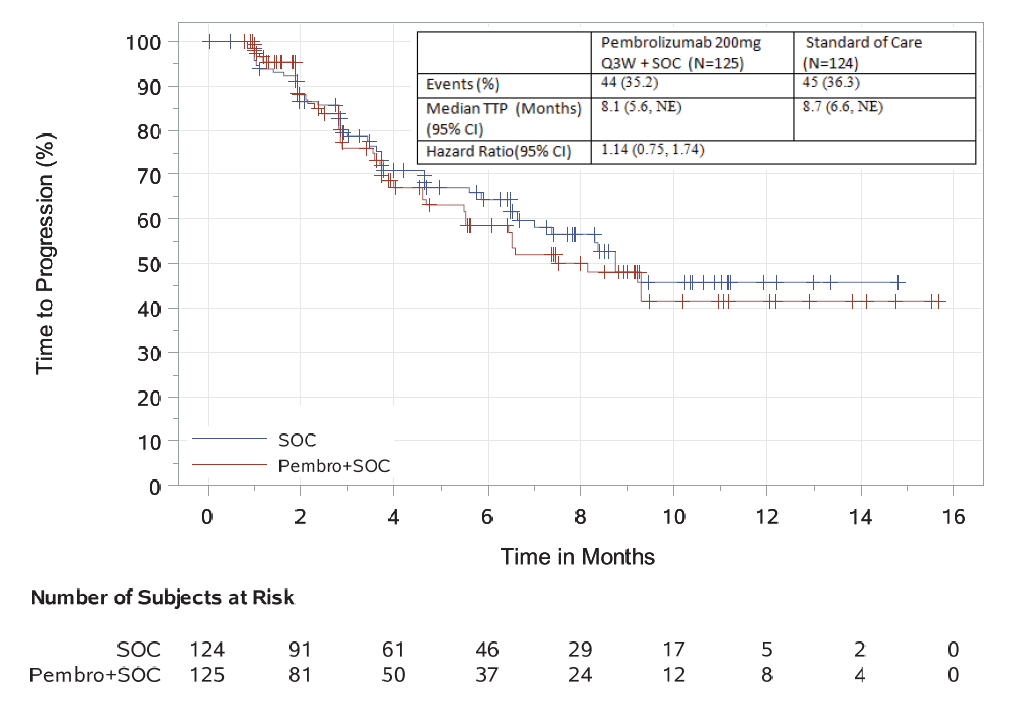
There was an 18% increase of severe, grade 3-5 toxicity (83% vs. 65%, investigational vs. control arm). The incidence of serious adverse events was 63% compared to 46% in the control arm. The following non-disease progression causes of death were identified in the pembrolizumab arm: myocarditis, Stevens-Johnson syndrome, myocardial infarction, pericardial hemorrhage, cardiac failure, respiratory tract infection, neutropenic sepsis, sepsis, multiple organ dysfunction, respiratory failure, and unknown.
KEYNOTE-185
KEYNOTE-185 is a phase 3, randomized, controlled trial of lenalidomide and low-dose dexamethasone with or without pembrolizumab in patients with newly diagnosed patients with multiple myeloma who are ineligible for autologous stem cell transplant.
The data cutoff date of June 2, 2017, was used for these analyses. There were 301 randomized patients included in the analysis. The median follow-up was 6.6 months. For overall survival, there were 19 deaths on the pembrolizumab-containing investigational arm, and 9 deaths on the control arm. The hazard ratio of the pembrolizumab-containing investigational arm compared to the control arm was 2.06 (95% CI: 0.93, 4.55), more than doubling the relative risk of death compared to the control arm.
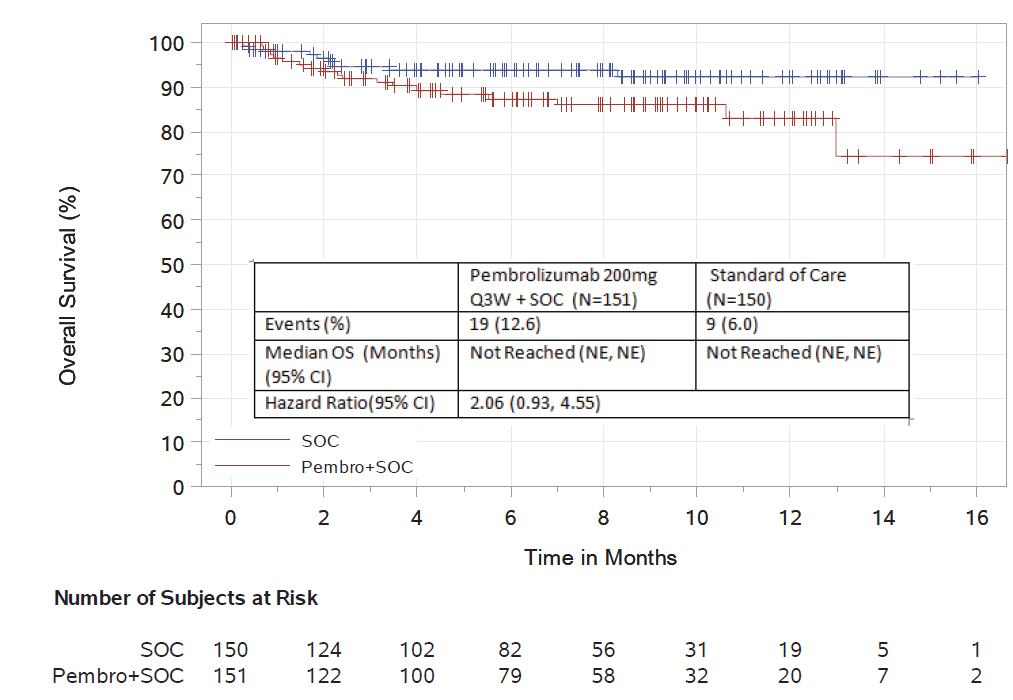
Additional efficacy analyses based on a June 2, 2017 data cutoff date demonstrated an objective response rate of 64% in the investigational arm compared to 62% in the control arm. In an exploratory time-to-progression analysis, a hazard ratio of 0.55 (95% CI: 0.20, 1.50) was observed.
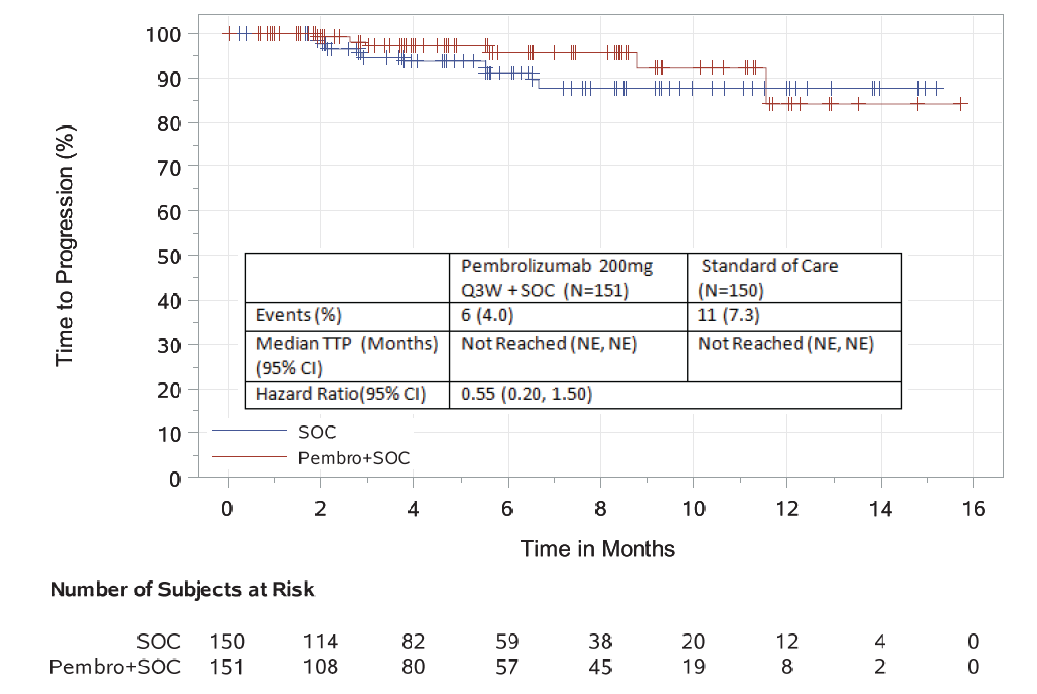
In the safety analysis based on a June 2, 2017, data cutoff date, there was a 22% increase of severe, grade 3-5 toxicity (72% vs. 50%, investigational vs. control arm). The incidence of serious adverse events was 54% compared to 39 % in the control arm. The following non-disease progression causes of death were identified in the pembrolizumab arm: intestinal ischemia, cardio-respiratory arrest, suicide, pulmonary embolism, cardiac arrest, pneumonia, sudden death, myocarditis, large intestine perforation, and cardiac failure.
In collaboration with the FDA and as a service to our members, ONS provides updates on recent FDA approvals and other important FDA actions (e.g., updated safety information, new prescribing information) pertaining to therapies for patients with cancer. This allows the agency to inform oncologists and professionals in oncology-related fields in a timely manner. Included in the FDA updates is a link to the product label or to other sites for additional relevant clinical information. In supplying this information, ONS does not endorse any product or therapy and does not take any position on the safety or efficacy of the product or therapy described.





