FDA Issues Warnings to Fraudulent Cancer Treatment Companies

On April 25, 2017, the U.S. Food and Drug Administration (FDA) issued warning letters to 14 companies that were selling fraudulently marketed products claiming to prevent, diagnose, treat, or cure cancer. In total, the companies produced more than 65 products that have been sold in the United States without FDA approval.
Disease Symptoms Most Likely to Predict Recurrence of Early-Stage Melanoma
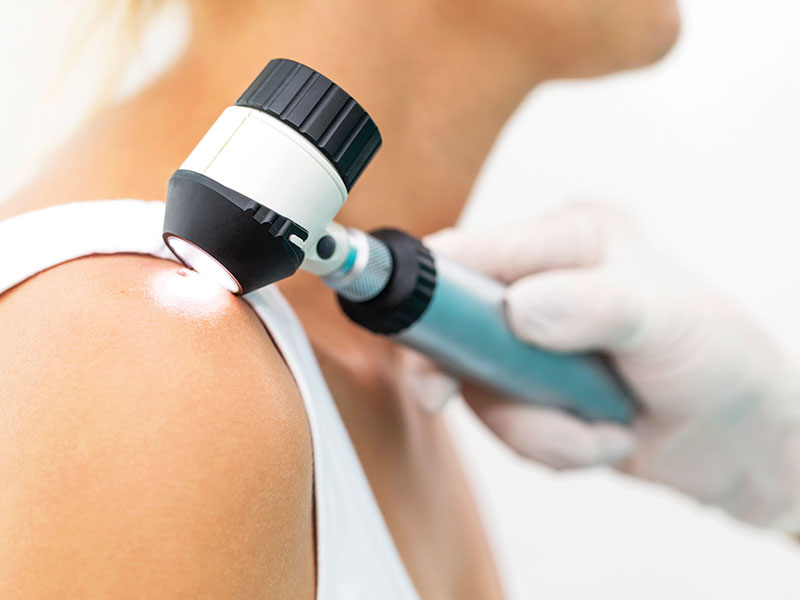
Patients and healthcare providers are most likely to detect recurrence of early-stage melanoma based on symptom reports rather than routine imaging tests, according to the results of a study published in the Journal of the American College of Surgeons.
- Read more about Disease Symptoms Most Likely to Predict Recurrence of Early-Stage Melanoma
- Add new comment
How Do I Move Standards of Care Into Practice?

What Are ONS’s Recommendations for Safe Handling of Hazardous Drugs?
Research suggests that healthcare workers who handle hazardous drugs may experience acute effects such as skin rashes or more chronic effects including adverse reproductive events and malignancy. This has led numerous government agencies to make recommendations regarding the safe handling of hazardous drugs.
- Read more about What Are ONS’s Recommendations for Safe Handling of Hazardous Drugs?
- 4 comments
- Add new comment
GOP to Potentially Revisit Healthcare Legislation; New State Funding Will Combat Opioid Epidemic; FDA Reaches Reauthorization Fee Deal for New Drugs, Devices

As the first 100 days of the Trump administration ends, the White House is still pressing Congress to revisit healthcare legislation—one of Trump’s main campaign promises.
- Read more about GOP to Potentially Revisit Healthcare Legislation; New State Funding Will Combat Opioid Epidemic; FDA Reaches Reauthorization Fee Deal for New Drugs, Devices
- Add new comment
Lori Brown Becomes ONS’s First Chief Experience Officer
Patients Treated for Early-Stage Breast Cancer Report Severe Side Effects

Nearly half of patients treated for early-stage breast cancer report at least one severe side effect, according to the results of a study published in Cancer.
- Read more about Patients Treated for Early-Stage Breast Cancer Report Severe Side Effects
- Add new comment
ONS Names Ellen Carr as Incoming CJON Editor
ONS Leaders Discuss Society’s Changing Role and Focus in Cancer Nursing Research
- Read more about ONS Leaders Discuss Society’s Changing Role and Focus in Cancer Nursing Research
- Add new comment
The Case of the Immunotherapy Inquiry

Jay is a 62-year-old man with newly diagnosed, stage IIIA (T3, N1), unresectable, non-small cell lung cancer (NSCLC) that tested negative for ALK, EGFR, and KRAS mutations. Additionally, PD-L1 (programed death receptor ligand) expression was less than 30%. Jay is symptomatic with a persistent cough, unintentional weight loss, and fatigue.





