Senate Republicans Unveil Replacement Healthcare Bill; Single-Payer Healthcare System Would Have High Price Tag; FDA Commissioner Comments on 2016 Youth Tobacco Survey Results

On June 22, 2017, Republican senators unveiled their version of the bill repeal and replacement bill for the Affordable Care Act, known to most as Obamacare. The Senate bill looks similar to the House-backed healthcare bill passed in May 2017. Central to the Senate’s bill are proposed cuts to Medicaid expansion, along with eliminating a net investment income tax that impacts higher earners. The proposed bill provides more tax subsidies for lower-income individuals than its sister bill from the House of Representatives, but it’s still expected to raise costs for poorer Americans.
- Read more about Senate Republicans Unveil Replacement Healthcare Bill; Single-Payer Healthcare System Would Have High Price Tag; FDA Commissioner Comments on 2016 Youth Tobacco Survey Results
- Add new comment
How NCI Is Training the Future Cancer Research Workforce
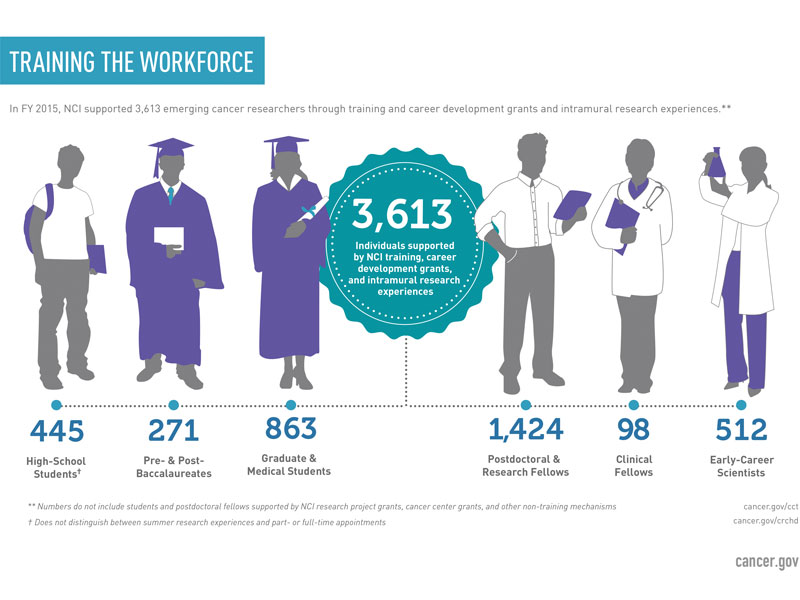
To ensure that future cancer research is of the highest quality, the National Cancer Institute (NCI) is committed to developing the best scientific minds. NCI training and funding opportunities cover a broad spectrum of disciplines for individuals at various stages in their careers, ranging from high school and graduate students to scientists, clinicians, and healthcare professionals.
FDA Approves Betrixaban for Extended Duration Prophylaxis of Venous Thromboembolism

On June 23, 2017, the U.S. Food and Drug Administration (FDA) approved betrixaban (Bevyxxa, Portola) for the prophylaxis of venous thromboembolism (VTE) in adult patients hospitalized for an acute medical illness who are at risk for thromboembolic complications due to moderate or severe restricted mobility and other risk factors for VTE.
- Read more about FDA Approves Betrixaban for Extended Duration Prophylaxis of Venous Thromboembolism
- Add new comment
FDA Approves Dabrafenib and Trametinib Combination for Metastatic NSCLC with BRAF V600E Mutation

On June 22, 2017, the U.S. Food and Drug Administration granted (FDA) regular approvals to dabrafenib and trametinib (Taflinar® and Mekinist®, Novartis Pharmaceuticals Inc.) administered in combination for patients with metastatic non-small cell lung cancer (NSCLC) with BRAF V600E mutation as detected by an FDA-approved test.
- Read more about FDA Approves Dabrafenib and Trametinib Combination for Metastatic NSCLC with BRAF V600E Mutation
- Add new comment
FDA Approves Rituximab Plus Hyaluronidase Combination for Treatment of FL, DLBCL, and CLL

On June 22, 2017, the U.S. Food and Drug Administration (FDA) granted regular approval to the combination of rituximab and hyaluronidase human (Rituxan Hycela, Genentech Inc.) for adult patients with follicular lymphoma, diffuse large B-cell lymphoma, and chronic lymphocytic leukemia.
- Read more about FDA Approves Rituximab Plus Hyaluronidase Combination for Treatment of FL, DLBCL, and CLL
- Add new comment
Combination Treatment Improves Survival for Advanced Melanoma

A combination of nivolumab and ipilimumab improved overall survival when compared to either drug alone, according to results from a recent study reported at the American Association for Cancer Research 2017 annual meeting.
Recent FDA Approvals Continue to Focus on Targeted and Immunotherapy

Oncology clinicians can expect to continue to see new targeted and immunotherapy drugs emerge as clinically approved agents in the fight against cancer. Five cancer-related U.S. Food and Drug Administration (FDA) approvals occurred in the first quarter of 2017; following are their indications for treatment and associated clinical implications. You’ll recognize that some of the agents were already FDA approved for other uses, but as clinical trials continue and new data emerge, clinical use is expanding to other disease sites and indications.
ACA Could Potentially Become Expanded Medicaid; Smoking More Prevalent in Low Socioeconomic Individuals; Provider, Patient Communication Still Needs Improvement

In Washington, DC, the healthcare debate rages on. Currently, Republican senators are working behind closed doors to modify and change the House-passed American Health Care Act (AHCA). As it stands, the AHCA is the replacement plan for the Affordable Care Act (ACA), known to most Americans as Obamacare. While legislators continue to debate in Washington, the insurance marketplace carries on. United Healthcare recently announced its departure from the ACA’s marketplace exchange, another in list of insurance companies that have chosen to leave.
- Read more about ACA Could Potentially Become Expanded Medicaid; Smoking More Prevalent in Low Socioeconomic Individuals; Provider, Patient Communication Still Needs Improvement
- Add new comment
Leadership Strategies for Nursing Excellence
The triple aim of healthcare is patient satisfaction, quality outcomes, and decreased costs. Navigation is the key to effective care delivery, said Regina Cunningham, PhD, RN, NEA-BC, FAAN, chief executive officer of the Hospital of the University of Pennsylvania, during the Endnote Session at the Oncology Nurse Advisor Navigation Summit.
What Rising Cancer Costs Are Doing to Patient Well-Being
The cost of cancer has increased substantially over the years and is continuing to trend upward. During a session at the Oncology Nurse Advisor Navigation Summit, Yousuf Zafar, MD, MHS, an associate professor of medicine and public policy at Duke Cancer Institute, gave some facts and figures on cancer costs and how these are impacting patient well-being.





