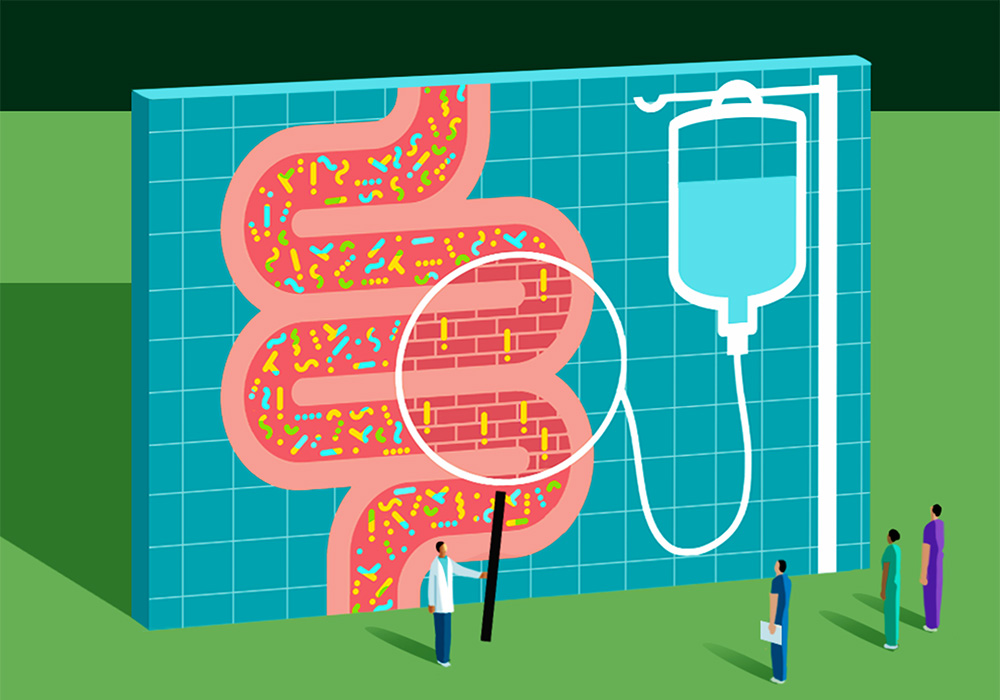
Our gut microbiome—the diverse community of microorganisms, including bacteria, archaea, viruses, and eukaryotes such as fungi, that inhabit the gastrointestinal (GI) tract—can affect our entire body’s health. Most people’s microbiomes have a healthy balance of organisms that stay contained in the GI tract because of the lining’s tight epithelial cell junctions. But from the gut, bacteria are capable of generating a range of vitamins, synthesizing amino acids, and breaking down and metabolizing indigestible carbohydrates. And a growing body of evidence—including studies conducted by oncology nurse scientists—is uncovering how the gut microbiome can influence a patient’s response to cancer treatment and their overall outcomes.

ONS member Zahra Amirkhanzadeh Barandouzi, PhD, RN, assistant professor at the Emory School of Nursing in Atlanta, GA, is part of a research team led by ONS member Deborah W. Bruner, PhD, RN, FAAN, that is investigating the associations between the gut microbiome and symptoms in patients receiving cancer treatments. She said that the numerous bacteria in the intestines generate antimicrobial substances, known as bacteriocins, and compete for nutrients and attachment sites along the gut lining.
“This activity effectively hinders the colonization of pathogens in the intestinal environment,” Barandouzi said.
ONS member Catherine Cherwin, PhD, RN, assistant professor at the University of Iowa College of Nursing in Iowa City, said that although studies are still emerging and many connections have yet to be identified, microorganisms may provide insight into cancer risk and side effects. For example, Cherwin said that Clostridioides difficile may be related to severe diarrhea and Helicobacter pylori may have a connection to stomach cancer.

Treatment Outcomes
The gut microbiome may influence how well an individual responds to cancer treatments, Cherwin said. She was part of a research team that used samples from people with lung cancer and healthy controls to look at the relationship among the microbiome, immunotherapy treatment response, and treatment-related adverse events. Cherwin said the team found that the composition of the gut microbiome of patients with cancer who had a better response to treatment had an enrichment of Clostridiales and a depletion of Rikenellaceae.
“We also found that patients with cancer who did not experience immunotherapy-related adverse events had an enrichment of Bifidobacterium and Desulfovibrio,” Cherwin said. “While these are preliminary results, other studies have also identified bacteria that may influence how well a patient responds to cancer treatment.”
Symptoms and Side Effects
Research also is starting to explore how the gut microbiome can influence symptoms in people with cancer, Cherwin said. Clinicians can predict to some degree who will have severe GI symptoms based on emetogenicity ratings and known toxicities of chemotherapy, but they haven’t been able to fully explain the biologic basis for those symptoms.
“I think we have all seen two essentially identical patients receiving identical cancer treatment, but one patient will have the expected symptom burden and complete their therapy as planned while the other person experiences symptoms far more severe than what was anticipated and either has to dose reduce or discontinue treatment,” Cherwin said. “I believe that the second patient is experiencing more harmful changes in their microbiome as compared to the first person and that we can identify individual bacteria that might be responsible for the severe GI symptoms.”
Cherwin cited a recent study in which her team that found that women with breast cancer receiving chemotherapy had an enrichment of Lachnoclostridium and Oscillibacter and a depletion of Prevotella 9, Akkermansia, Lachnospira, and Lachnospiraceae NK4A136 compared to healthy controls. Any imbalance like this one can set patients up for more severe symptoms and side effects.
Barandouzi’s research corroborates that. She said that patients receiving radiation therapy have increased abundance of opportunistic microbes, such as Clostridium cluster XIVa, Proteobacteria, and Gammaproteobacteria, and decreased abundance of beneficial microbiota like Faecalibacterium, Lachnospiracea, Oscillibacter, Roseburia, and Streptococcus. The microbiome of patients receiving chemotherapy undergoes the same phenomenon with decreases in beneficial microbiota like Firmicutes, Actinobacteria, Bacteroides, Bifidobacteria, and Clostridium clusters IV and XIVa and increases in opportunistic microbes like Proteobacteria and Enterobacteriaceae.
“These changes can lead to fatigue, depressive symptoms, pain, sleep disturbance, and cognitive impairment,” Barandouzi said.
She said that during one of their studies, the research team at Emory demonstrated that patients treated with radiation therapy had lower microbial diversity and more severe symptoms. In contrast, patients with less severe symptoms had a higher abundance of the healthy bacteria Ruminococcus and Lachnospiraceae NK4A136, which are associated with reduced inflammation.
Patients with severe side effects or compounding symptomatology may have trouble completing their treatments, and the relationship to the gut microbiome is a promising emerging area of study. Barandouzi said that more investigations are needed using advanced technologies.
“While we can categorize the gut microbiome as healthy or opportunistic, we need to consider that using the current method (16S rRNA), we just have a picture of what is going in the gut (imbalance in the gut microbiome composition, known as microbiome dysbiosis),” she said. “By using more advanced methods, such as shotgun metagenomic sequencing, we can have a more clear-cut distinction between healthy and opportunistic bacteria.”
The Microbiome in Clinical Practice
Barandouzi advised that nurses can use information about the gut microbiome to evaluate and track treatment-related symptoms that can affect patients’ quality of life.
“Healthcare providers can provide guidance on promoting a healthy gut microbiome through adopting a nutritious diet and a healthy lifestyle, as well as incorporating prebiotics and probiotics,” she said. She recommended that nurses familiarize themselves with the potential risk factors for gut microbiome dysbiosis, as numerous cancer treatments heighten this risk.
Cherwin agreed. “Looking at research describing the functions of those bacteria, we see that they are responsible for key aspects of gut health like maintaining a healthy mucus barrier, anti-inflammatory effects, and production of short-chain fatty acids.” Because it is a relatively new area of discovery, she encouraged nurses to keep abreast of the latest findings and look to their professional organizations to develop recommendations once the evidence base is established.
“There has been a growing body of evidence showing that prebiotic dietary interventions, probiotic supplements, and fecal-microbiome transplants have varying degrees of success in preventing or managing microbiome-associated GI symptoms,” Cherwin said. “However, the evidence can be inconsistent and is relatively new, so evidence-based treatment recommendations from panels of experts will be helpful in guiding how to safely implement these in practice.”
Both experts urged nurses to be prepared to discuss microbiome-influencing interventions with their patients so they can have the option of talking with their provider about safely using them to reduce or prevent severe cancer treatment-related symptoms.
“Information about the microbiome has made it to mainstream media and social media sources, meaning that inaccurate or even dangerous health recommendations can spread,” Cherwin said, adding that microbiome interventions can be an asset to cancer care but should always be done under a provider’s supervision.