Genetic Disorder Reference Sheet: FH Tumor Predisposition Syndrome
Fumarate hydratase (FH) deficiency occurs in individuals who have homozygous (https://www.ons.org/genomics-taxonomy/genome-foundations#heterozygous) pathogenic variants in the tumor suppressor FH gene. The condition results in (https://www.ncbi.nlm.nih.gov/books/NBK1506/) poor feeding, failure to thrive, hypotonia, lethargy, and seizures. Development is severely delayed (https://www.ncbi.nlm.nih.gov/books/NBK1506/), and individuals are often nonverbal, unable to walk, and die in early childhood.
Most individuals with heterozygous (https://www.ons.org/genomics-taxonomy/genome-foundations#heterozygous) pathogenic variants in the FH gene are healthy but have (https://doi.org/10.1136/jclinpath-2021-207830) autosomal-dominant (https://www.ons.org/genomics-taxonomy/mode-inheritance) FH tumor predisposition syndrome. FH is a cancer susceptibility biomarker (https://www.ons.org/genomics-taxonomy/biomarkers) because it predicts an individual’s risk for developing (https://www.ncbi.nlm.nih.gov/books/NBK1252/) cutaneous leiomyomata, uterine leiomyomata (i.e., fibroids), and renal tumors. Some families also have pheochromocytomas and paragangliomas.
Clinical Characteristics
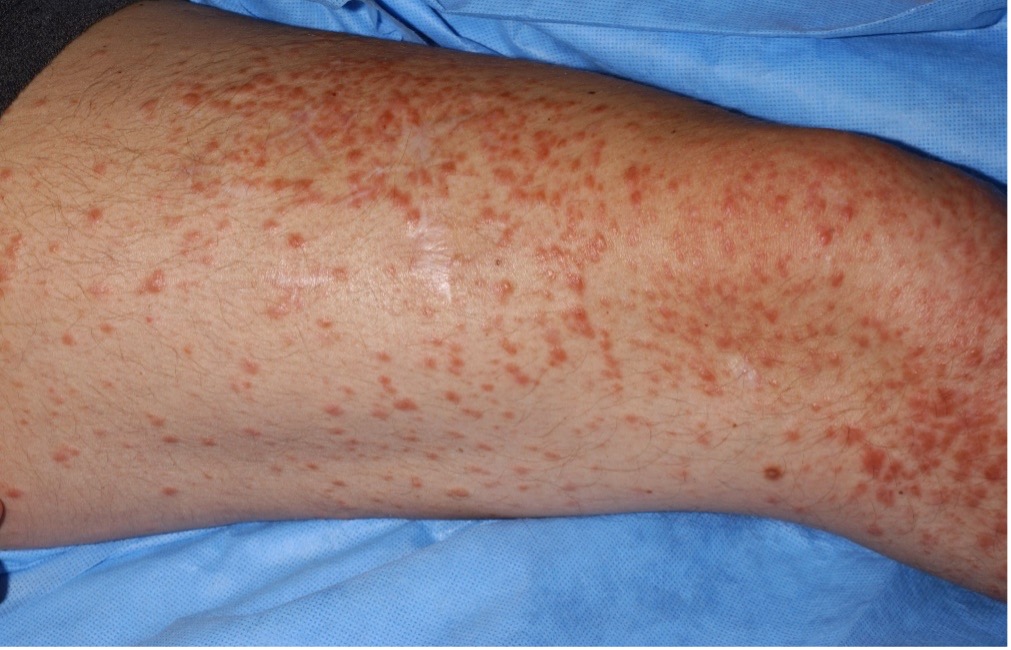
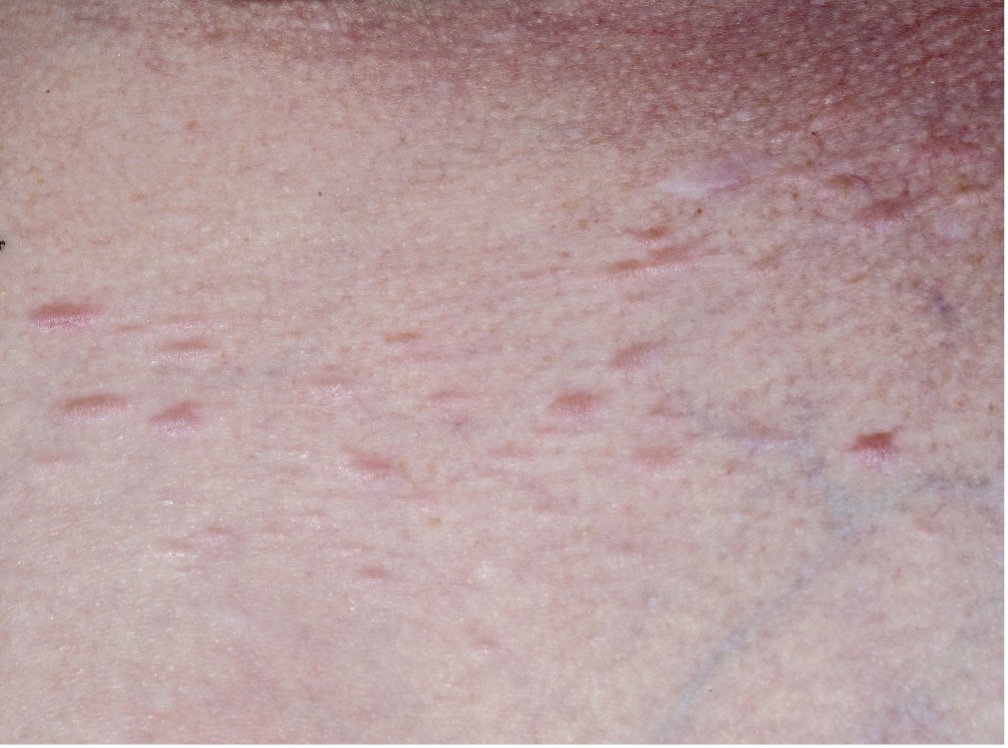
Cutaneous leiomyomata (see images in sidebar) are benign smooth muscle skin tumors that are flesh-colored to light brown papules distributed over the trunk, extremities, and occasionally face. Lesions typically appear around age 30 and continue to increase in size and number with age. They may manifest as single lesions, clusters, or widely disseminated papules.
Uterine leiomyomata tend to be numerous, large, and highly penetrant (https://www.ons.org/genomics-taxonomy/genome-foundations#penetrance) (42%–100% penetrance (https://doi.org/10.1111/aogs.14248)). Most patients are diagnosed at age 18–57 after experiencing irregular or heavy menstruation and pelvic pain, and 53% elect to have a hysterectomy by age 40. Fibroids show loss of FH staining and prominent, inclusion-like eosinophilic nucleoli.
Renal tumors are usually unilateral, solitary, and aggressive, with an estimated risk of 15%–21% (https://doi.org/10.1111/aogs.14248). The variety of pathologies include papillary, tubulocystic, and collecting duct carcinoma. Although the mean age of diagnosis is 40 years, children are also affected. FH-associated renal tumors are linked with poor survival because of their clinical aggressiveness and propensity to metastasize despite a small primary tumor size. Because of that, FH is considered a prognostic biomarker (https://www.ons.org/genomics-taxonomy/biomarkers).
Screening, Early Detection, and Monitoring
Recommendations are continually evolving but currently include (https://www.ncbi.nlm.nih.gov/books/NBK1252/):
- Cutaneous leiomyomata: Begin full annual skin exams at age 20 to assess disease extent and evaluate for changes.
- Uterine leiomyomata: Conduct annual gynecologic exams starting at age 20.
- Renal tumors: Begin screening at age 8 (https://www.nccn.org/) using magnetic resonance imaging with contrast, preferably with 1–3 mm slices through the kidney. Computed tomography with contrast may be used as an alternative. Renal ultrasound is not recommended for primary surveillance because of its low sensitivity to detect small lesions. Promptly refer suspicious renal tumors, including indeterminate lesions and questionable or complex cysts, for further evaluation and follow-up.
Treatment and Management
Recommendations are also continually evolving but currently include (https://www.ncbi.nlm.nih.gov/books/NBK1252/):
- Cutaneous leiomyomata: Surgical excision may be used for a solitary or a few symptomatic lesions, but recurrence rates are high. Lesions may also be treated with carbon dioxide laser, cryotherapy, or electrodessication.
- Uterine leiomyomata: Surgical options include myomectomy and hysterectomy, depending on the size of the lesion and need for fertility preservation. Conduct a careful histologic examination before surgery to differentiate between atypical smooth muscle neoplasm and leiomyosarcoma.
- Renal tumors:
- Because of FH-related renal cell cancer’s aggressiveness and poor prognosis, it may require earlier and possibly more extensive surgical excision (https://nccn.org/) than other renal cancers.
- Targeted therapy is undergoing active research, including treatment with vascular endothelial growth factor receptor tyrosine kinase inhibitors or mammalian target of rapamycin inhibitors, making FH pathogenic variants a predictive biomarker (https://www.ons.org/genomics-taxonomy/biomarkers). Fumarate accumulation in FH-deficient cells may lead to a defect in homologous recombination double-strand DNA break repair, which may be appropriate for treatment with poly ADP-ribose polymerase inhibitors (https://www.ncbi.nlm.nih.gov/books/NBK1252/).
Patient Education
Early detection requires patients to self-monitor their health. Oncology nurses can help individuals with FH pathogenic variants understand symptoms to report to their healthcare team immediately:
- Renal cancer: hematuria, lower back pain, or a palpable mass
- Pheochromocytoma and paragangliomas: uncontrolled blood pressure, rapid or irregular heartbeat, headaches, increased sweating feelings of panic, fear or anxiety, hearing loss, loss of balance or coordination, or loss of bowel or bladder function
Nursing Implications
Because renal cancer screening begins at age 8, genetic testing is recommended at that age as well. First-degree relatives have a 50% chance of having a pathogenic variant. Testing partners of individuals with heterozygous variants who are considering conception provides a more accurate risk of the child having FH deficiency. Credentialed genetics professionals can assist families with that process. In addition to education on screening and symptoms to report promptly, patients require psychosocial support and a consistent team of providers to implement detection measures.