NIH Diversity Research Program Gets New Chief Medical and Scientific Officer

A pioneer and internationally recognized expert in translational genomics and precision medicine will guide the scientific vision, strategy, and data collection for the next phase of the National Institutes of Health’s (NIH’s) All of Us Research Program, the agency announced. In November 2021, NIH appointed Geoffrey Ginsburg, MD, PhD, to serve as the program’s chief medical and scientific officer.
- Read more about NIH Diversity Research Program Gets New Chief Medical and Scientific Officer
- Add new comment
Bolster Your Team for Better Well-Being

Nurse burnout can only be addressed at the health system level, and beginning in your unit is one way to get that process started.
Education, Insurance, and Marital Status Linked to Disparities in Survivorship Care Plans

Older cancer survivors who have lower levels of education, are uninsured, or are widowed, divorced, or separated are less likely to receive survivorship care plans (SCPs), researchers found. They reported the results of their study in Supportive Care in Cancer.
- Read more about Education, Insurance, and Marital Status Linked to Disparities in Survivorship Care Plans
- Add new comment
Mental Health Teams Build Programs That Prioritize Staff Well-Being

Given the inherent nature of caregiving, burnout has been part of the nursing profession since the beginning. As the complexities of the profession increase, so do nurses’ vulnerability. Oncology nurses have multiple physical, emotional, and mental demands, which, if left unaddressed, can lead to burnout, compassion fatigue, and moral distress.
Winning Team Develops Solution to Increase Preceptor Development in Third ONS Hackathon in Collaboration With ONCC

The Oncology Nursing Society (ONS) launched its third ONS–ONCC Hackathon™ in November, designed to create innovative solutions for current and future nurses in the delivery of high-quality, evidence-based cancer care in all possible settings.
- Read more about Winning Team Develops Solution to Increase Preceptor Development in Third ONS Hackathon in Collaboration With ONCC
- Add new comment
Make Subcutaneous Administration More Comfortable for Your Patients
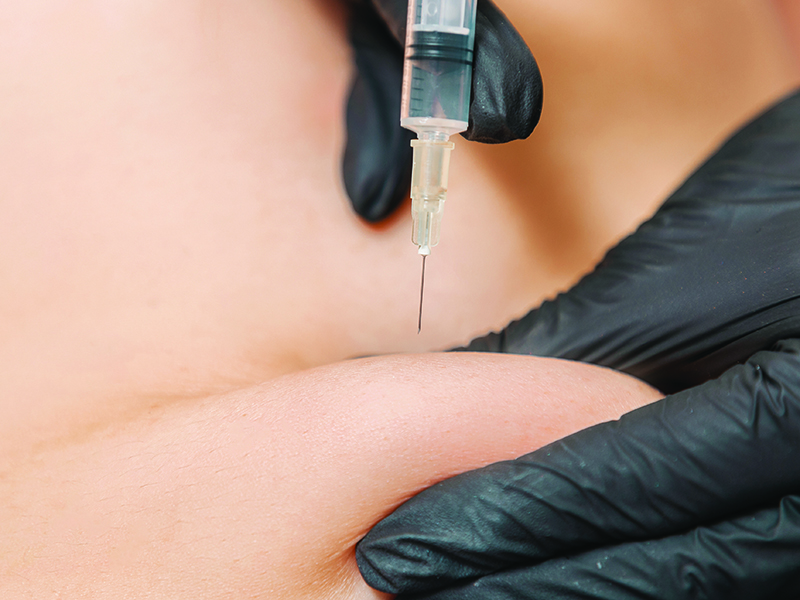
Much of oncology nursing education focuses on IV administration of systemic therapies because for years, that was the only route. Until 2004, only two cancer therapies were approved for subcutaneous (SC) administration, and just nine others were added through 2012. However, as more familiar IV therapies get SC counterparts, including the more recent approvals of four high-volume monoclonal antibodies (mAbs), infusion nurses are using them more regularly in practice.
Two Years Later, We Are Older and Wiser

As we turn the calendar to the new year, it feels a bit like the film Groundhog Day. Information about the COVID-19 pandemic—and related topics like masks, vaccination, and protecting ourselves and our patients from infection—still dominates the news and the literature. Many areas are struggling with surges in winter cases, and we all feel the personal loss of friends and family, colleagues, and patients and the toll it has taken on our profession. It is, without doubt, a constant presence in our lives.
Show the World Your Beautiful Mess

Embracing—not judging or hiding—our flaws and vulnerabilities makes us happier and more relatable, a research-supported concept called the beautiful mess effect. What we think are negatives or weaknesses, others see as courageous. Showing vulnerability can lead to stronger relationships, increased self-esteem, and better mental health.
Liver Cancer Diagnoses See Geographic, Racial, Income Disparities

The urban-rural disparity in hepatocellular cancer (HCC) diagnoses is widening, researchers said, particularly in certain racial, income, and age groups. The authors reported their study results in Clinical Gastroenterology and Hepatology.
Oncology Drug Reference Sheet: Amivantamab-Vmjw
After clinical trials demonstrated a 40% overall response rate with 63% of responses lasting six months or more, on May 21, 2021, the U.S. Food and Drug Administration (FDA) approved amivantamab-vmjw (RybrevantTM) for adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) exon 20 insertion variants, as detected by an FDA-approved test, whose disease has progressed on or after platinum-based chemotherapy.





