This is the second in a series detailing some of the general factors to consider around patient adherence to oral medications, and ways to support patients receiving neratinib.
Case Study: Adherence to Neratinib Treatment
(Note: This is a hypothetical patient case)
AS is a 58-year-old woman who was diagnosed at age 55 with a right infiltrating ductal carcinoma of the breast, identified as grade III, ER and PR positive, and HER2 positive. She had bilateral mastectomy, and tumor size was noted to be 2.8 cm. She had a positive sentinel lymph node. She was staged at IIB (pT2, pN1, M0). Her left breast was noted to have usual ductal epithelial hyperplasia and a 1.2 cm fibroadenoma. AS’s genetic testing revealed a BRCA1 mutation (BRCA1 c.3748G>T [p.Glu1250*]), which may confer an increased risk for breast cancer in the range of 46%–87%, and 39%–63% risk for ovarian cancer by age 70. As her patient history shows, she has some risk factors for nonadherence, including no support at home and some financial issues. Throughout her care, the team worked to communicate and prepare AS to help ensure adherence as much as possible.
Patient History
Genitourinary history: She is post hysterectomy and right oophorectomy for benign disease.
Comorbidities: Seasonal allergies and chronic sinusitis; anxiety and depression (taking paroxetine and lorazepam per primary care provider); history of treated H. pylori.
Family history: No suspicion for hereditary mutations was revealed.
Social history: She is a smoker, about half a pack per day with a 20-year history. Upon diagnosis, she was trying to quit smoking with nicotine patches. She is divorced, lives alone, and has two grown children who live out of state. She has inadequate finances and receives government healthcare insurance.
Treatment
AS was referred to an oncologist after bilateral mastectomy; therefore, neoadjuvant therapy was not given. According to the National Comprehensive Cancer Network (NCCN) guidelines (2018), neoadjuvant treatment is an acceptable option. This can help patients avoid axillary lymph node dissection if they are responding well to therapy.
Chemotherapy plus trastuzumab is an NCCN category 1 recommendation. An alternate regimen with the addition of pertuzumab could have been considered. AS received six cycles of TCH (taxotere, carboplatin, and trastuzumab) with pegfilgrastim support for three months. She experienced mild grade 1 peripheral neuropathy. She also had short-term grade 1 diarrhea post chemotherapy. TCH is a preferred NCCN regimen, particularly in those with cardiac risk factors. Because she did not receive neoadjuvant therapy with pertuzumab, it was also not given as postsurgical adjuvant treatment.
Following chemotherapy, AS completed a year of trastuzumab given every three weeks. During adjuvant therapy with single-agent trastuzumab, she also completed adjuvant radiation to the breast and axilla. During chemotherapy and radiation therapy, she had mild gastroesophageal reflux disease and gastritis symptoms. Echocardiograms during trastuzumab therapy were within normal limits. After she completed radiation therapy, AS began adjuvant treatment with aromatase inhibitor therapy. In November, AS’s oncologist recommended a new HER2-targeted oral therapy called Nerlynx® (neratinib) to be taken for one year for further adjuvant treatment.
Neratinib Treatment Journey
Indications and usage
Neratinib is a kinase inhibitor indicated for the extended adjuvant treatment of adult patients with early stage HER2-overexpressed/amplified breast cancer, to follow adjuvant trastuzumab-based therapy.
The oncology advanced practitioner explained to AS that HER2-overexpressed breast cancer has increased HER2 signaling that stops natural cell death and promotes abnormal, excessive growth of breast cancer cells. Even with adjuvant treatment with trastuzumab, about 25% of women will still have a breast cancer recurrence. Neratinib blocks abnormal signaling and results in cancer cell death, decreasing the chance of breast cancer recurrence. AS agreed to the therapy and neratinib was prescribed at 240 mg once a daily for one year.
The oncology nurse educated AS on neratinib, providing written information and a medication reminder calendar. The nurse explained that diarrhea usually begins within the first two weeks of therapy and that patients will often experience diarrhea within the first month. She said that preventing diarrhea in the first two months is important, when it is most likely to be severe. AS began prophylactic treatment for diarrhea on the first day of treatment with neratinib. If diarrhea occurs, careful management is necessary to prevent dehydration and other complications. The nurse instructed AS in signs or symptoms to report to the oncology healthcare team and assured her that dosage adjustments could be made to manage diarrhea, if needed, so she could remain on her treatment.
The CONTROL trial results suggest a loperamide prophylaxis regimen to prevent issues that could occur because of diarrhea. The CONTROL trial is an ongoing, international open-label trial of patients with stage 1–3c HER2-positive breast cancer that focuses on the prevention of diarrhea in patients receiving neratinib. The trial has four arms to examine different dosing regimens of loperamide along with the combination of loperamide and budesonide or colestipol. Data from the CONTROL trial demonstrate that prophylaxis with loperamide for the first two cycles of therapy (56 days) significantly decreases the incidence, severity, and duration of diarrhea associated with neratinib.
Strong communication is another route to help increase patient adherence to the medication. Specific written instructions given to AS to manage diarrhea, with the goal to help maintain adherence by controlling side effects, were as follows:
Weeks 1–2 of neratinib therapy: loperamide 4 mg three times per day
Weeks 3–8 of neratinib therapy: loperamide 4 mg twice a day
Weeks 9–52 of neratinib therapy: loperamide 4 mg as needed (not to exceed 16 mg per day)
AS was instructed that if diarrhea was not controlled with loperamide, then other medications could be prescribed, and was educated on when to call the oncology healthcare team. Colestipol is a sequestrant believed to target the bile acid malabsorption also seen in preclinical models of neratinib-induced diarrhea. Data on the control of diarrhea from the addition of colestipol is not yet mature, but preliminary data suggest that adding colestipol will prove beneficial if a patient requires a more extensive diarrhea treatment regimen.
The clinic pharmacist reviewed the patient’s current medications for potential drug-drug interactions. AS used occasional over-the-counter proton-pump inhibitors for gastroesophageal reflux disease. Taking neratinib and omeprazole together could decrease neratinib levels, thus potentially decreasing efficacy and possibly affecting adherence. Therefore, she was advised to avoid those types of medications. She was also instructed that if she used any antacids, she should separate dosing with neratinib by at least three hours. The pharmacist also discussed with her the need to check with the pharmacist or healthcare provider if any other medication was prescribed or advised for her because of the risk of interactions.
AS began neratinib in November. Within the first week, she began having constipation (grade 2) and was instructed to hold loperamide for one day and then resume at one tablet three times a day. She was told that if any diarrhea began, she was to resume originally prescribed dosing of loperamide. A few days later, she resumed the original dose of loperamide because of grade 2 diarrhea. She also reported mild nausea without vomiting. She had prochlorperazine at home and she was advised to take it 30–60 minutes prior to neratinib. With any adverse reaction, accurate grading of toxicities enables the oncology provider to make proper decisions about dose interruptions, dose reductions, or drug discontinuations (see Tables 1 and 2).
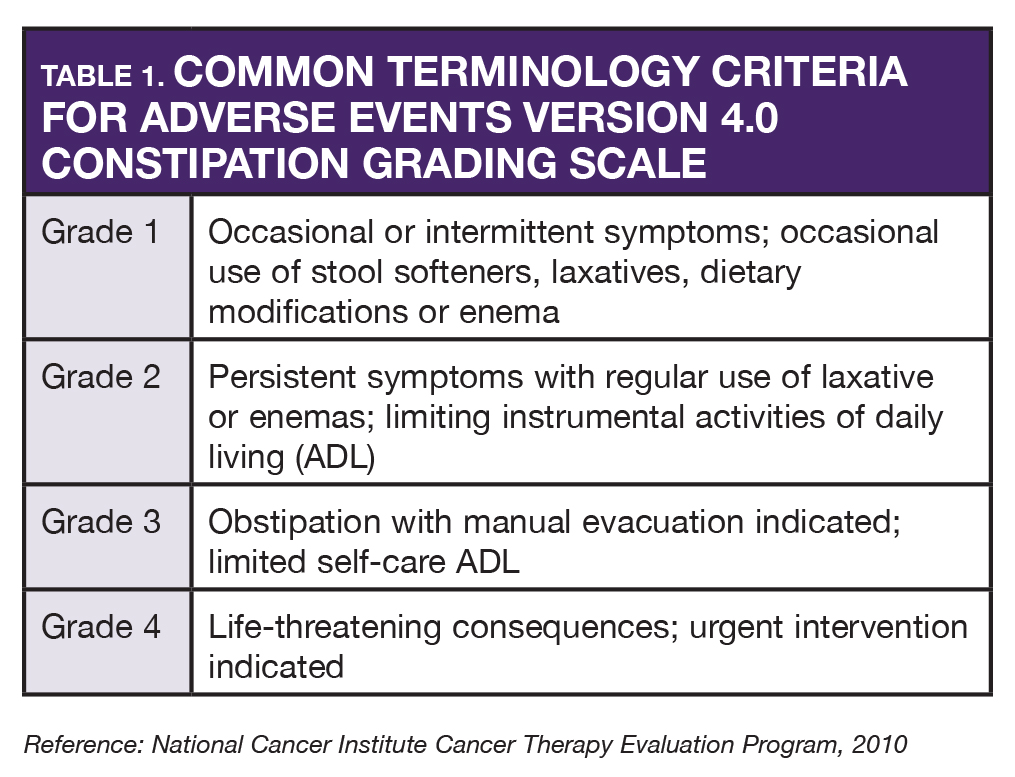
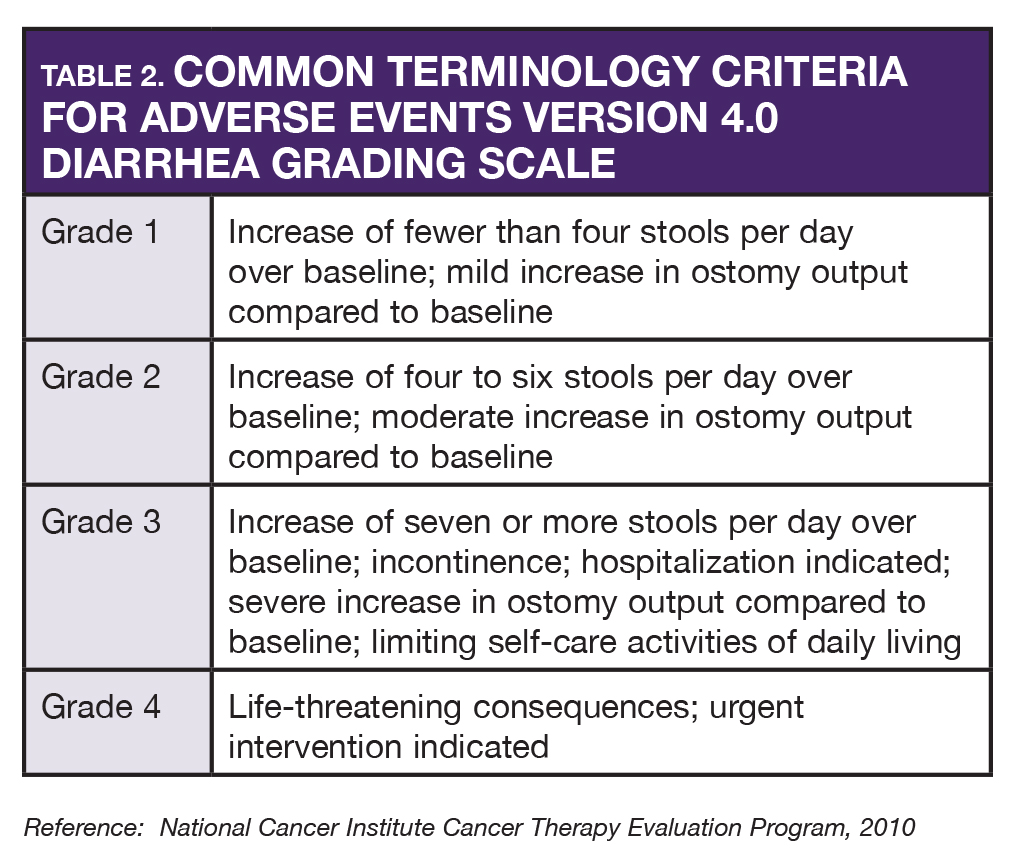
During periodic follow-up calls, AS noted that her diarrhea was well controlled with loperamide and not interfering with her normal activities. At three months of therapy she occasionally had some mild diarrhea that required loperamide.
AS was scheduled for lab tests to monitor liver function monthly for the first three months, and no abnormalities were found. A dose reduction is needed for severe hepatic impairment, but no dose modifications are required for mild to moderate hepatic impairment. At baseline AS had no hepatic dysfunction, and thus was started at full dose. Further liver function monitoring was scheduled at three-month intervals for her duration of neratinib therapy due to the precaution for potential hepatotoxicity with neratinib. Dose reductions could be indicated if an issue was to develop, and a grade 3 liver abnormality would require temporary withdrawal of neratinib until resolved.
During therapy, at every healthcare visit, and on every follow-up call, AS was asked about adherence to therapy.
Providers should assess both adherence and tolerance of therapy. Healthcare personnel on triage must be aware of what therapy patients are taking and potential side effects. Providers must be proactive rather than reactive to toxicity. Early recognition and management of toxicity is vital to keeping patients on effective treatment.
Current Situation
After a year of therapy with neratinib, AS is doing well. All of her lab results are within normal limits and her diarrhea is well controlled. Her adherence has been strong, as far as the nurses have been able to assess. Continued strong communication has helped the patient remain on the medication through the issues that occurred.
Read the other articles in this series:







