New guidelines and consensus recommendations for managing immune-related adverse events (irAEs) from checkpoint inhibitors are available from several key cancer and immunotherapy organizations: a collaboration between the American Society of Clinical Oncology and National Comprehensive Cancer Network, and a separate consensus recommendation from the Society for Immunotherapy of Cancer. ONS contributed to the development of both sets of guidelines.
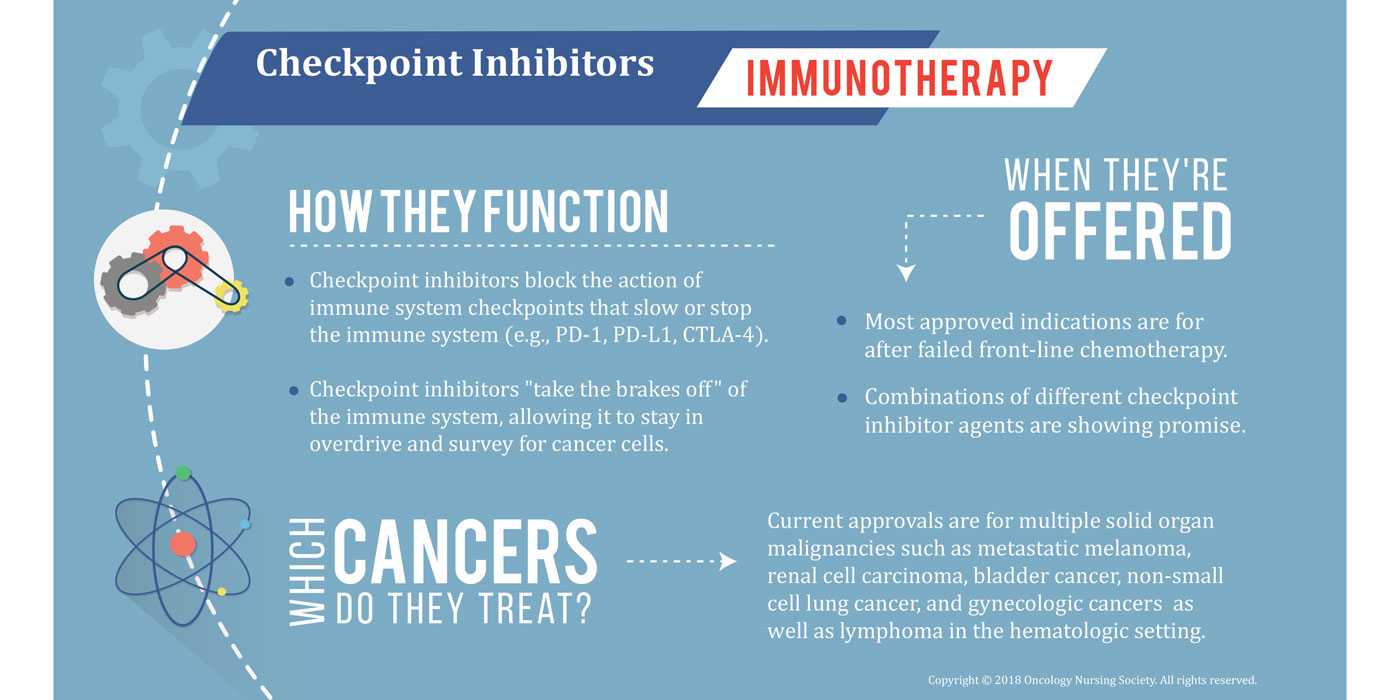
Checkpoint inhibitors are a widely used class of immunotherapy agents that essentially “take the brakes off” a patient’s immune system and allow the body to target cancer cells for destruction. That increased immune activity, while effective against cancer, can also result in irAEs that could eventually cause dose reductions or treatment delays. Additionally, the irAEs may appear similar to side effects from other cancer therapies, but they must be managed very differently when caused by immunotherapy.
When compared to chemotherapy side effects, irAEs usually have a delayed onset but prolonged duration, and early identification and intervention are key to effective treatment. They commonly occur in the skin, gut, endocrine, lung, and musculoskeletal systems and more rarely cause cardiovascular, hematologic, renal, neurologic, and ophthalmologic events.
Oncology nurses can use the guidelines and recommendations to help develop evidence-based institutional procedures for managing irAEs in patients receiving checkpoint inhibitors.